In this article, we explore a basic workflow process of additively manufactured implants. By no means is this text an ultimate guideline of the exact process, as different parts and assemblies require different procedures and equipment. With this said, the main objective is to offer a general overview of the manufacturing of a surgical implant, making special emphasis on what differentiates this process from a regular 3D-printed part in non-medical workflows.
The Analysis Process: Planning the Solution
As with any other problem that needs solving, creating an implant is creating a solution. In this case, a patient has an internal component that need replacement or repair. This internal component may be a bone, part of a blood vessel, or even to create a separation between two tissues for them to not “fuse”. And of course, all of this starts with the surgeon.
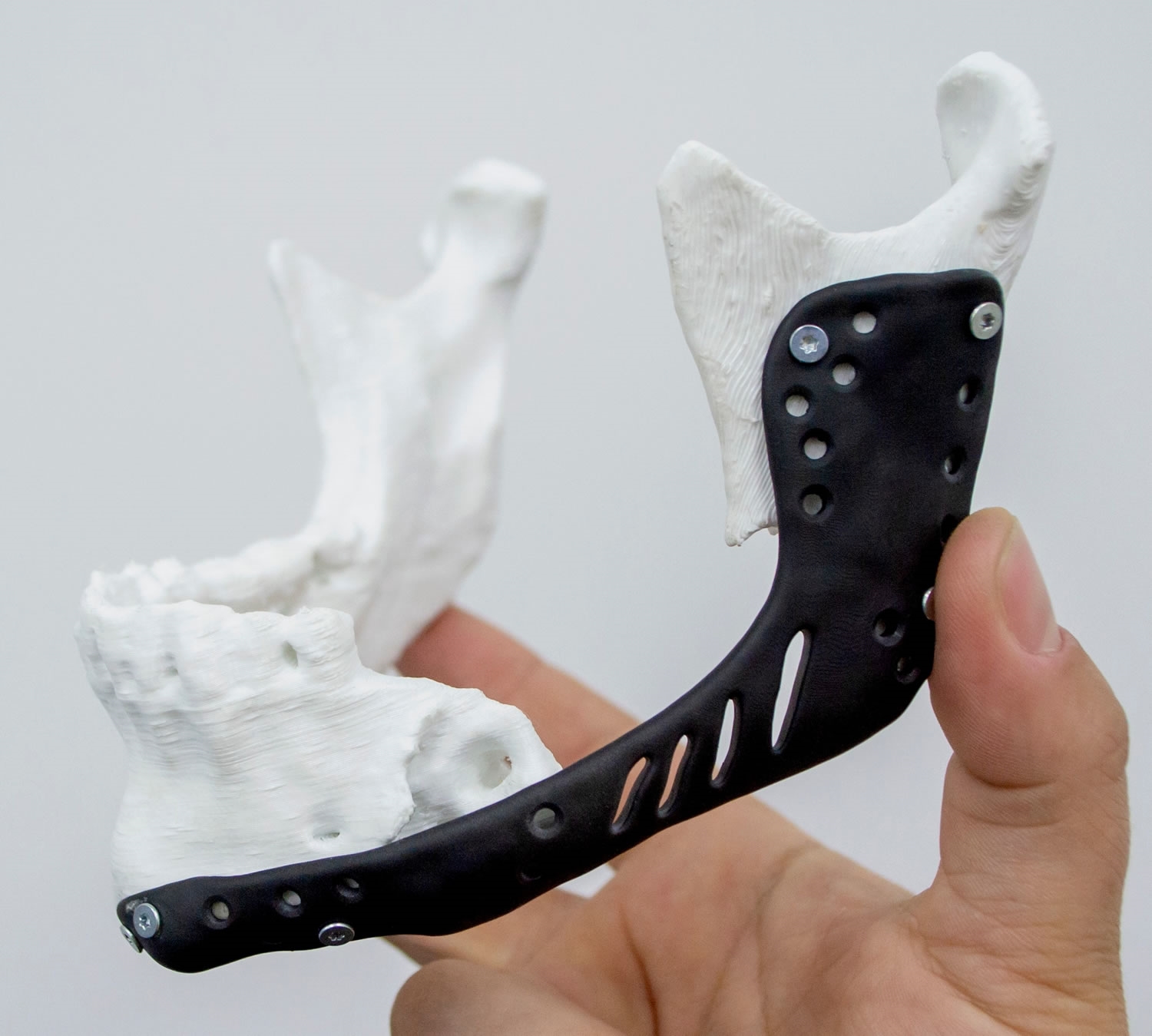
For the sake of simplifying this article, let’s take an example: A patient walks into a hospital with severe trauma to his lower jaw. After scanning the patient, the imaging shows “the ramus” shattered into pieces beyond reconstruction. The surgeon suggests that the best way to proceed is by having the shattered part removed, and an implant created to replace the damaged part of the bone.
Then it’s time for the engineering team: after receiving the data from the PET scan and the biocompatibility material guidelines from the surgeon, they decide that the best way to proceed is to FDM print (Fused Deposition Modeling) the shattered part out of PEEK (Poly-Ether-Ether-Ketone) with a simulated part out of a stiff UV resin using DLP (Digital Light Processing) to assess attachment points and provide the surgeon with the ability to simulate the surgical process beforehand and try different approaches to the surgery.
Designing an Implant
Now that our implant has been planned, it's time for the engineering team to recreate the model. By first recreating the lesioned bone in DLP, and viewing the 3d model from the PET scans, they can plan the design of the implant.
Additive Manufacturing, From CAD to Physical Part
Here we explore two of the most common additive manufacturing technologies that have been mentioned and which are currently available for creating implants: FDM and DLP. Other methods, including SLM (metal sintering) and jet binding, are also available.
The DLP process is straightforward. The scanned data is sent to the manufacturing team and printed in resin. The final model is then post-processed, inspected, and cleaned before being sent to the engineering design team for validation. At this point, we need to state that the surgeon has to be involved in all the processes. Being the “project owner”, he is responsible for inspecting and validating the parts for accuracy and conformity.
Now to the FDM process. Medical-grade FDM additive machines have a key point that differentiates them from a regular hobby and industrial 3D printers, and it's the conformity standards they must follow. These will require being manufactured by a company ISO-13845 certified as per medical devices standards, and ensuring each of its components (extruder, frame, heated chamber, etc) are free from lead and other contaminants.
In our example, the implant must be manufactured out of PEEK. This engineering grade polymer is known for its chemical resistance, high stiffness, high glass transition, and its biocompatibility, making it one of the most suitable materials for invasive implants. The downside of PEEK is that it requires high-temperature extruders, a heating chamber that can go up to 160ºC (320°F), and a heated bed that reaches more than 200ºC (392°F), and if support material is required, that also has to abide by the high temperature requirements of PEEK. This makes PEEK one of the most difficult materials to 3D print, requiring a high-end FDM machine.
Post-Processing The Parts
Now that we have a part, it’s time for post-processing it, from sanding with different grit grades, sandblasting, and coating. In this case, PEEK doesn't accept any type of coating due to its chemical resistant nature, but if our implant were made out of titanium, a Titanium Nitride coating would probably be the best way to proceed. Again, this decision falls on the medical and engineering design team.
Cleaning, The Key to Success
This is one of the key parts that differentiates this process from all other industrial processes. The part or assembly resulting from this workflow is going to be inside a person's body, therefore, it is mandatory to abide by class III risk medical device standards for cleaning and sterilizing the parts. A great deal of literature can be found on how to clean and disinfect implants in preparation for the sterilization process. The chemicals and cleansing agents are the star of the show.
Such complex parts resulting from additive manufacturing processes require a new breed of chemicals and applicators, as often they result in organic shapes, with multiple cavities and lattice structures to maximize adherence to organic tissues.
Chemtronics offers Tri-V vapor degreaser solvents that do exactly that. Having such a cost-effective solution for cleaning the printed implants before the sterilization is a game-changer for the process at hand. The reasons are:
- Low toxicity for the operator and surrounding equipment.
- High penetration cleaning performance.
- Safe for the implantable device, chemically and structurally.
A safe way to integrate the Chemtronics product line and receive an expert diagnosis of your cleaning workflow for implantable devices is to contact Chemtronics directly. They can even test it by replicating it at their facility and evaluate the results for you.
Sterilization and Logistics
The sterilization process is pretty straightforward. It can either happen at the manufacturing level, medical care center, or both. This is no different from the current procedures, therefore the hospital or clinic can easily integrate the workflow, at both levels, cleaning and sterilization, provided their facilities respond to local and ISO standards for sterilization circuits and machinery.
Conclusion: A Long Way Ahead
The manufacturing of implantable devices and parts coming from additive manufacturing workflows is a game-changer for the industry. Offering better implants that are tailored for the patients, therefore maximizing adaptability to the patient and their quality of life afterward. But this doesn’t come without its caveats; manufacturing medical devices and implantable devices require high-performance cleaning.
Ask A Technical Question
Stay up-to-date on Chemtronics news, products, videos & more.