Speakers:
- Pierce Pillon – Senior Field Engineer, ITW Contamination Control Electronics
- Kevin Pawlowski – Application Specialist, ITW Contamination Control Electronics
- Patrick Oliver – Sales Manager, Baron Blakeslee
Table of Contents (click to view subject)
Introductions & Overview
Kevin Pawlowski:
Well, welcome everybody to the Expert's Guide to Vapor Degreasing. Now I'm Kevin Pawlowski, Marketing Manager for ITW Contamination Control Electronics. I'll be your moderator today? I'm also an Application Specialist, so I know my way around vapor degreasing but not like our speakers today.
First of all, we have Pierce Pillon. He's the Senior Field Engineer for ITW and formerly the lab manager. So he has definitely had unique insight to the solvent side of this topic. Pierce has been with the company representing Chemtronics and Techspray for about 18 years. Pierce is very active with SMTA and IPC, and has been on the board helping to draft industry specifications that many of you have to live by every day.
And then also, we have Patrick Oliver, who is the Sales Manager from Baron Blakeslee, a top name in vapor degreasing equipment. Patrick has been in the precision cleaning industry for almost 30 years, and will be taking you through the nuts and bolts side of the topic. So welcome, guys.
This is the agenda of what we're covering today. So we're going to go through an overview. Pierce is going to take you through an overview of vapor degreasing, and a brief history, and explain how vapor degreasing works. So just an overview for those of you who are not familiar with the technology at all.
Then Patrick is going to go through a deep dive into the equipment itself. So the equipment overview, the process setup.
And then Pierce is going to circle back into the solvent side of the business, because they go hand-in-hand. And that's why this is such a nice opportunity to hear to get experts on both sides of the business. And so he's going to take you through the cleaning solvent challenges and how to select a solvent. Some of the key things you have to keep in mind for selecting a solvent. And then we're going to go through the questions as I mentioned.
History of Vapor Degreasing
Pierce Pillon:
A little history on vapor degreasing. It was developed in the late 19th century, most of the published accounts list Germany. So that's what we'll go with. The technology migrated over to the US during the 30s, and it just revolutionized critical cleaning. I mean, at that point, there really wasn't critical cleaning as we know it today, but it was a fairly large step up from what they were doing. During World War II, obviously, it did flourish during that time with all of the added manufacturing that was going on here in the US.
The first solvents that were predominantly used were perchloroethylene, or people know it as Perc or PCE, and also TCE or trichloroethylene. Other common solvents that have come and gone over the years are the various Freons, HCFC-141b, AK225, all of those are no longer available. So over the years, there's been a lot of good ones that have come and fell out of favor with the government.
Vapor Degreasing Today
Pierce Pillon:
Vapor degreasing today. Well, continuing pressures from the various regulatory agencies has driven the equipment manufacturers to refine their technologies, come up with newer technologies, newer ways to capture emissions, a lot of different ways to improve the environmental footprint, let's say, of the equipment.
And at the same time, the chemistries have changed over the years, again, due to a lot of the regulatory requirements, and it seems like every time we find a good solvent it goes on for a while and then, obviously, the government finds some way to take it away from our toolbox. So it's an ever changing world with both the equipment manufacturers and the chemistries suppliers.
It's in almost every industry you can think of. And I've just listed a few here: automotive, aviation, aerospace, electronics, medical, fine machining, firearms, you name it. If it needs to be cleaned, you will find vapor degreasing in that industry.
Operation Principles of Vapor Degreasers
Pierce Pillon:
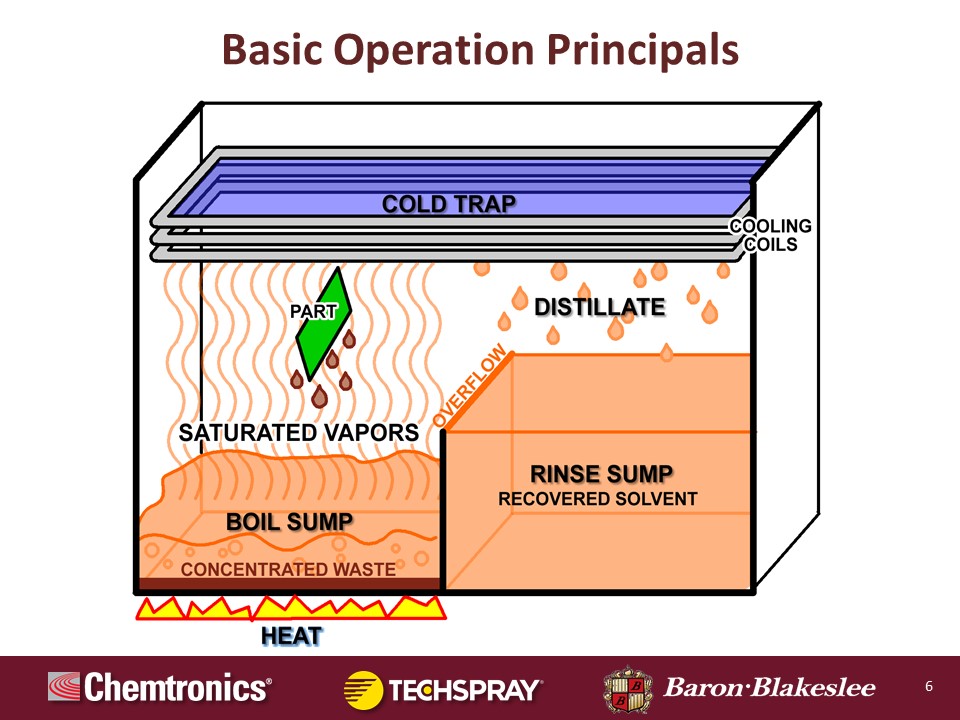
So let's just go through some real basic operation principles. And what we're going to do is we're just going to use the basic two-sump system: a boil sump and a rinse sump, and just one primary set of coils without going too deep into the weeds. And as you can see here, this is just kind of a flow of how this works. And we'll take you through in the next few slides exactly how each section works.
Okay, the boil sump. The heat is applied to the bottom of the boil sump which includes the liquid. It starts out everything is virgin in these units. The rinse sump solvent, the boil sump solvent are all brand new and virgin, never been used. So the heat is supplied to the boil sump, and all of the cleaning is done over the boil sump, so it can contain all of the concentrated waste. Now, as this liquid boils it vaporizes and it goes up and it covers all that empty space between the boil sump and the cold trap or the cooling coils up on the top. So I want to take you through a little bit about how this part is cleaned.
This is the main cleaning zone, what they call the vapor zone. And as this part is lowered either individually or in a basket of parts, those parts are cooler than those hot vapors. And this is the essence of how it works. Those vapors come up, hit these cool parts, and it condenses to a liquid back onto these parts which dissolves the contaminants, and the continued liquid condensing on there rinses the contaminants off, and then they're captured in the boil sump.
Now once this part reaches equilibrium temperature with those vapors, the cleaning stops, the dripping stops, the condensation stops, that cleaning cycle is over. Hopefully your part is cleaned by that point. But if it's not, that's okay, we have a plan B. All you have to do is raise that part or basket of parts back up into that cooler area, let them cool off and create that temperature delta again, and then lower them back into the saturated vapors and it will start a new cleaning cycle.
Let's go right to the cooling coils. The coils run a refrigerant through there of some sort. Some of the very, very old models will run water through it. Most of them are running a refrigerant of some sort now, HFC-134a. Some of the ones with secondary coils are running 404A, which is considered as a subzero chiller. Those coils up in that cold trap, those vapors will condense just like it did on the part, it will condense on those coils and then it drips off into a collecting trough.
Now as it goes into the trough, that actually empties out into the rinse sump or the distiller receiver is what it's called in a lot of cases. And so that is a recovered solvent, it is clean. And as that fills, it should be kept liquid full at all times. So as it builds it overflows or weirs back over into the boil sump to replenish that. So it's just a cycle, over and over and over.
Vapor Degreaser Cleaning Methods
Pierce Pillon:
Okay, as you can see there's a lot of different cleaning methods that are available to the operator. And a lot of that depends on number one, what options your unit has, does it have a wand? Does it have ultrasonics? Just various different options that are available on the degreaser unit itself, but primarily is dependent on the part geometry, the contaminant itself, the chemistry you're using, and the heat mass of that part. And the heat mass of that part is actually very critical.
You can have a small part or you can have an extremely large part, and that's going to determine the time of your cleaning cycle. The large parts are going to take longer for that temperature delta to decrease, and so the part equalizes with the vapor, so you'll get a longer cleaning cycle at that point. Smaller part won't take very long. It could just be a matter of a minute or so and that part reach equilibrium, and that cycle is over.
Vapor Degreaser Equipment Overview
Patrick Oliver:
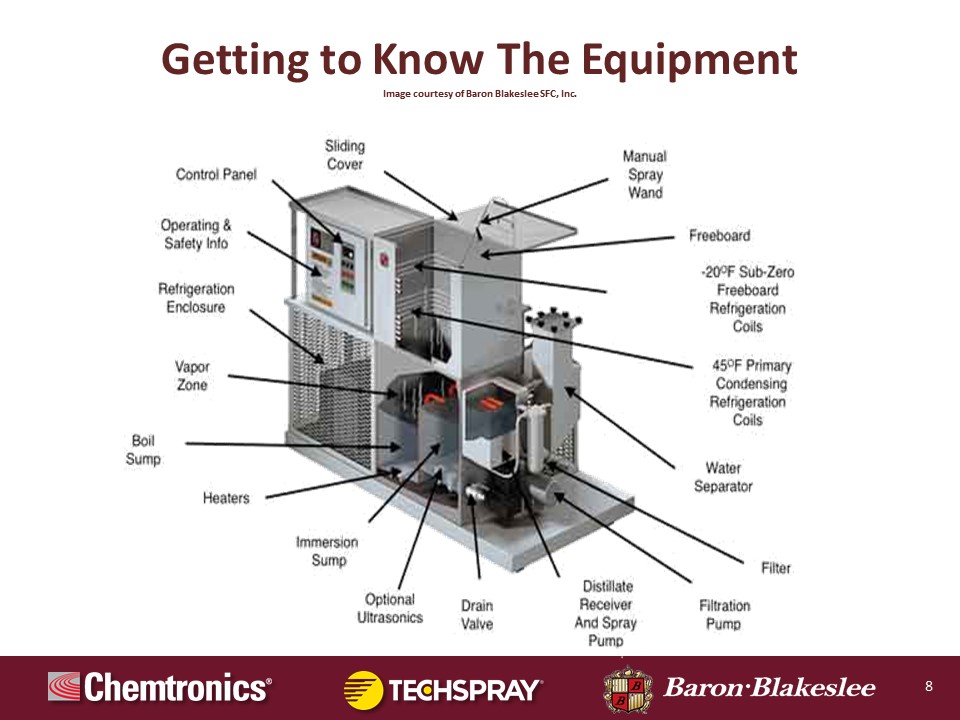
What you're looking at here is a cutaway of a conventional open top vapor degreaser. And you can see all the parts here they're labeled, and it is somewhat busy. But there are relatively few parts, it's a fairly simple system, again, as was described previously in the graphic you saw. I'll just kind of go through that solvent cycle again.
The boiling sump is the tank on the left where the heat is applied and the phase of the solvent changes from liquid to vapor. The vapor is going to float up inside the tank and come in contact with the primary condensing system. This is the lowermost set of coils. And as Pierce mentioned, these are the evaporator coils from a refrigeration system. They're cool. Again, normally run those around 45 degrees Fahrenheit. So this extracts the energy that's supplied by the heaters, and then the condensing liquid falls by gravity into a trough below. There's a gutter if you will, that runs around the perimeter of the internal area of the tank collecting the condensing solvent, and then we flow into the water separator.
The water separator is an external tank in this case, and it fills with the distillate. And then the water is less dense than the solvent and it can be separated by gravity. It floats on top, you open a valve, drain the water out. From the water separator it flows into a tank we call the distillate receiver. This is where a distilled solvent that has been dried free of water accumulates and it may be sprayed onto part surfaces with a manual spray wand. This is a common feature in open top vapor degreasers. Not every machine has it, but many units do have a manual spray wand that the operator can use, controlling with a foot switch to direct low pressure, low flow spray of clean fresh distilled solvent onto part surfaces to enhance the cleaning of areas of geometric complexity or if you need to focus on a certain soil that you see on the surface, the wand can help with that.
The spraying is done below the vapor line. And the vapor line is right at the midpoint of the primary condensing coil set. So if there's four wraps, the vapor will be fixed between say, wrap two and three, it's held there in tension. And then the condensing solvent, the fresh clean distilled solvent overflows from the distillate receiver into the immersion sump, which is the clean sump. So parts may be placed in there. Generally that sump is filtered to remove insoluble particulate, and the cleaning in that sump may be enhanced by the use of ultrasonics which can be beneficial in providing the scrubbing action where the ultrasonic cavitation is focused specifically on areas of geometric complexity, blind holes, capillaries, porosity. So it can help to sort of scrub soils away from the surface.
The tank you have it in is fresh, clean, distilled solvent. Now one of the merits of this is that the temperature in that immersion sump is slightly colder than the zone of saturated vapor above it. So when you elevate parts out of the immersion sub, you pause them in that zone of saturated vapor, you'll get a final condensing rinse, a fresh, clean pure solvent. And this condensing rinsing action occurs until the part temperature warms to the end of the vapor. At the time when vapor and part temperature are equal, condensation stops, cleaning stops, the parts are dry. And then you can elevate them into the freeboard zone, which the freeboard is the uppermost set of coils. And again these are typically the evaporator side of an R-404A refrigeration system. They run at about minus 20 degrees Fahrenheit.
This particular degreaser has two refrigeration systems. The purpose of the upper freeboard refrigeration system is to create a very heavy, dense blanket of cold air that lays down on top of the solvent vapor to suppress it. It helps confine the vapor in the degreaser, but it also by virtue of the fact that air is very dense, can minimize the effects that drafts may have, potentially displacing solvent vapor when the cover's open.
So just to recap what I said, one of the benefits of doing that final condensing vapor rinse post-immersion, it adds repeatability and consistency to this process. The potential for the redeposition of soils in a vapor degreasing process is correctly maintained, properly operated, is virtually nil. In many other types of cleaning processes, take a water cleaning process for instance, where say you have like a conveyorised washer, a belt washer, if you will, where you're washing and rinsing and drying parts that move on a conveyor. You're constantly being challenged with keeping the detergent out of the rinse water, keeping the rinse water out of the drying stage. So it's difficult to prevent the redeposition of soils.
But consistency of that process may not be quite as high as you like, whereas in a vapor degreaser it's very consistent very repeatable, because you're always cleaning in fresh, clean pure distilled solvent. And the final part of that process that the parts see or come in contact with is clean vapor which is essentially pure virgin solvent.
So basically this is the layout of a vapor degreaser. One thing I will mention on open top degreasers we always use a sliding cover. That way when the cover is opened, you do not create a draft that can pull solvent out or you don't create a draft that can displace solvent when the cover closes. In the old days, degreasers had a lift off cover or a hinged cover, and that's a design we no longer use, we use the sliding cover. All the typically, equipment that we make as well as other manufacturers in this regard generally are compliant to the US EPA NESHAP regulations, which govern the design of vapor degreasing equipment. And these are features that impart efficiency and keep the solvent in the degreaser. So, that's essentially the layout of the open top.
Parts of a Vapor Degreaser
Patrick Oliver:
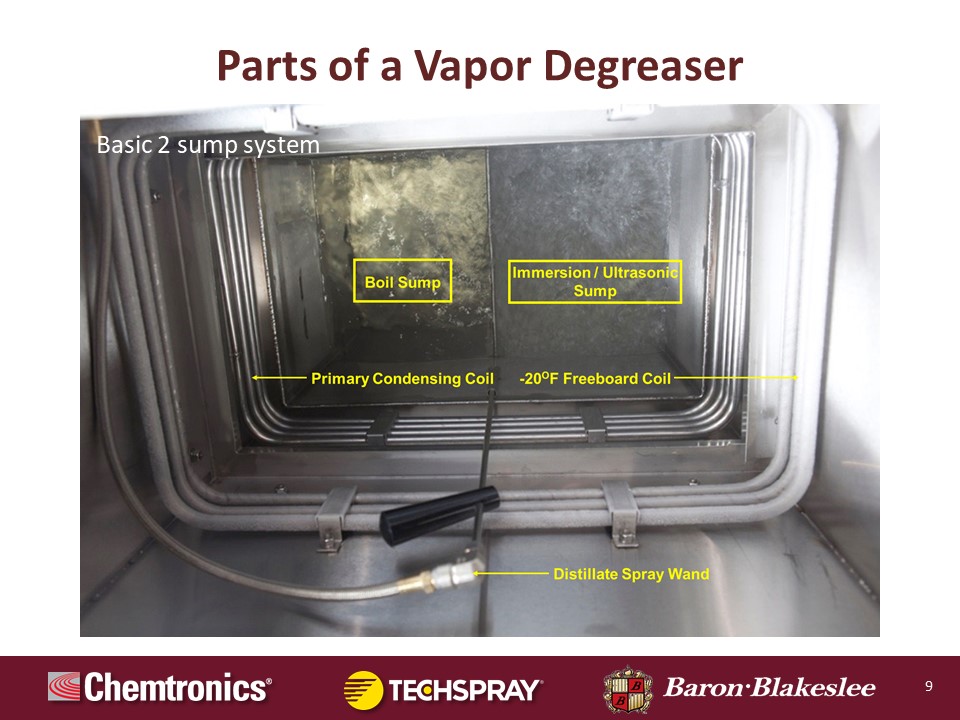
Now what we're looking at here this is an actual photograph of the interior of a vapor degreaser, an open top degreaser. And you can see there are two sumps. On the left there's the boiling sump. Again we're looking down into the tank. This is like the plan view of the system looking straight down into the tank. The boiling sump is on the left. Again that's where the heaters are located. That's where the energy is applied to boil the solvent, generate vapor, change the phase from liquid to vapor. All of the soluble contaminants, oils, grease, anything that will dissolve in the solvent eventually migrate to the boil sump, and they are sequestered there again where they cannot redeposit on parts.
The sump on the right is the clean sump. The immersion sump generally filtered to remove insoluble particulate. Often ultrasonics are installed in there to enhance cleaning. And as you're moving up into the plane of the photograph, what you can see there is the primary condensing coil. That's the lowermost set of coil. This again is where the load is applied by the heaters and where that load of energy is extracted. This is actually what's changing the phase of the solvent from a vapor back to liquid, thus confining it in the degreaser and providing the mode of force if you will, behind the distillation process, which ultimately purifies the solvent.
Then up above those coils is the secondary subzero freeboard refrigeration system. Again, running at minus 20. If you look closely, you can see a little bit of frost forming on that coil because it operates below the freezing point of water. Again, this is creating that heavy dense blanket of cold air that's laying down on top of the vapor suppressing it. And because those temperatures of the evaporator are so cold, we have to run a defrost cycle once an hour for a few seconds. We'll do a hot gas bypass on the refrigeration system, melt that frost. In other words, we want to keep those coils running at minus 20 Fahrenheit. If you allow the layer of ice to build up, suddenly now the temperature is 32 degrees Fahrenheit and efficiency has dramatically decreased. So that's a very important feature for solvent conservation.
In larger degreasers, sometimes the coils have a thinned geometry as opposed to being smooth walled. And in even larger open top degreasers we actually split the uppermost freeboard refrigeration system into two banks. So one defrosts while the other continues to cool. And then in the foreground, you'll see the distillate spray wand where it's accessible by the operator. They can pick up that wand, spray parts in the vapor, and again sort of enhance cleaning.
This is again an immersion degreaser, basically two sumps. Some variations of this technology do not have liquid immersion. It's just a true vapor degreaser where parts are processed only in the zone of saturated vapor. But this is I think by far the most common where you have a dual sump unit. So again, in some cases, you can immerse in the boil sump if you want to put the parts right in contact with the solvent which is the hottest, however it is the dirtiest. Sometimes if you're trying to soften things like wax or heavy grease, you may want to park the parts in the oil sump initially.
As Pierce mentioned, oftentimes parts are initially allowed to dwell in the vapor above the boiling sump for just a vapor degreasing process, a preclean if you will, prior to immersing in the clean sump. And you can do that. And then, that mitigates the contaminant loading into the boiling sump because the contaminants fall by gravity into the boil sump. You can transfer to the clean side, and that's where you get more of a precision cleaning or again, you can use it to remove particulate or enhance cleaning through the use of ultrasonics.
So the spray wand can be used either before or after immersion depending on where you're trying to preclean or just do a final clean. Again, the distillate that is dispensed by the spray wand is colder than the vapor temperature. So you can cool the parts down once more and provide an extra-long cycle where the parts are exposed to the final vapor rinse for a higher level of purity in the cleaning process. So there's a lot of versatility.
Can the Vapor Degreaser Spray Wand Disrupt the Vapor Blanket?
Kevin Pawlowski:
Hey, Patrick, a quick question on the spray wand. This is coming from an attendee. "It's my understanding from past experience that manual spray wands can disrupt the vapor blanket and create solvent loss.” What's your view on that option?
Patrick Oliver:
Spray, and sometimes we use fixed spray manifolds and vapor that are programmable that come on automatically. We've even done things like articulating spray wands, but the manual spray wand for one thing, the pump that powers the spray wand, we do not allow that pump to energize until we know that the vapor blanket is fully established. And we require that spray happen below the vapor line. However, it is manual and it is up to the operator, completely incumbent upon the operator to use it correctly and properly.
But to your point, you're right, spray does potentially increase emissions because you're creating turbulence in the vapor, you're spraying cold solvent, relatively cold solvent compared to the boiling temperature of the solvent and the solvent vapor, and the vapor does to some extent condense and coalesce on those droplets of solvent in the vapor. We try to provide spray systems that are very low pressure, very low flow with a nozzle selected with the intent not to atomize the solvent but to provide more of a, kind of a gentle stream.
We don't want to create an aerosol, we don't want to make this refrigerant going through an expansion valve. We don't want that. We just want it to kind of spritz. And it's not like you're at the carwash or you're blasting moss off your deck. I mean, this is a very, very low pressure low flow. But I think that manual spray is something that should be used judiciously, and maybe as a necessary part of a specific cleaning process. But you're right, you have to balance whatever benefits the spray may provide, versus the potential emissions that may be associated with it.
Vapor Degreaser Condenser Coils
Patrick Oliver:
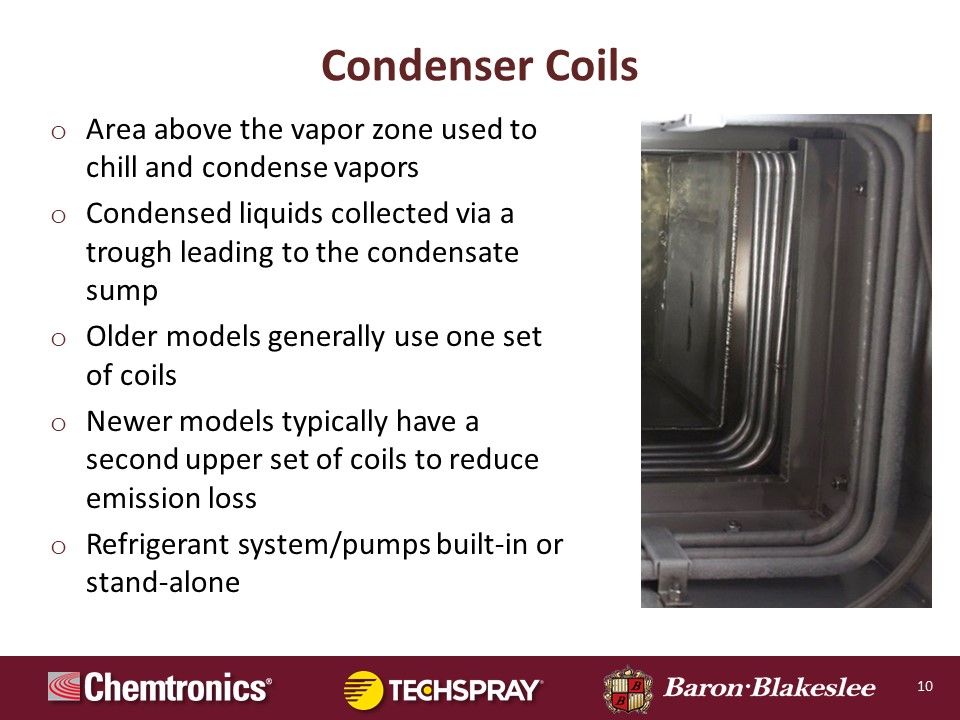
All right, so here we are with a slide of the condenser coils, and I kind of touched on this a little bit. Again, over to the right hand side you see a section of the degreaser. Again, the lower most coils are the primary condensing system, which is used to extract the energy supplied to the system by the heaters. The purpose there is to essentially confine the solvent in the degreaser. Older model machines may only have one set of coils, only that primary set. We make some very small machines like bench top size degreasers where the tank opening is very small. And in those we can get by with a single set of refrigeration coils by virtue of the fact that the tank opening is small.
When tank openings get larger, we have to go to a system where you've got the dual refrigeration system, where you have the uppermost set of the secondary subzero freeboard coils. Again, in most applications, these coils are the evaporator side of a refrigeration system. Again, R-134a for the primary, R44A for the secondary subzero freeboard.
When we get into much larger machines. Oftentimes, the primary cooling we use water, it's circulated. Some people use tap water, that's very inefficient. Water from a cooling tower like an evaporative cooling tower, but most commonly, it's water that's circulated through a hydronic chiller, or maybe a glycol water mixture. And just for the sake of efficiency on large degreasers, that's normally the way the primary condensing is accomplished with water. And again on larger machines, we'll be using Fin coils instead of the smooth wall.
But one of the things that's real benefit to that secondary subzero freeboard refrigeration system, the second set of coils, if you had two degreasers comparably equipped with the only difference being one was equipped with a secondary subzero freeboard refrigeration system, that degreaser so equipped would have solvent emissions approximately half that of the degreaser not so equipped. So this is a very important feature for solvent conservation, especially in the modern era where solvents that are fluorinated generally have higher vapor pressures, lower boiling temperatures, are generally more evaporative than the legacy solvents such as trichloroethylene, perchloroethylene. So from a perspective of mitigating impact to operator environment reducing process costs, the use of secondary subzero freeboard refrigeration brings tremendous benefits in that regard.
Vapor Degreaser Distillate Recovery Sump
Patrick Oliver:
And here we have the distillate recovery sump, also known as the clean sump or the immersion sump. Again, the solvent that is in this sump has already been through the process of evaporation, condensation, in other words distillation. It's been through a water separator to dry it, to remove the moisture, and it's constantly spilling into the sump. This sump is clean by virtue of the fact that clean, fresh distilled solvent is constantly flowing into it. There's a mass flow of solvent all around the degreaser from the process of boiling, evaporation, condensation, accumulation in this sump.
In other words, so if you're processing parts in here, and they've got soluble contaminants: oil, grease, solder flux, solder paste, it will dissolve in the solvent in this sump. And this sump is constantly being overfilled with fresh, clean, distilled solvent. So the soluble contaminants cascade across the weir, separating this from the boiling sump. So there's a constant displacement of soluble contaminants into the boiling sump. And through the process of dilution with fresh, clean, distilled solvent, this sump stays clean.
And when we design vapor degreasers we're sizing them to process a specific mass of a metal of given specific heat, steel, aluminum, whatever, per hour. But we're also taking into account attempting to maintain a specific distillation rate, as well as accounting for radiational losses of the metal and whatever. But for the most part, we provide an abundance of energy to process the mass of parts you need to process but also keep this sump clean, and to have a fresh, clean plentiful supply of vapor, because you've got to have vapor established to make the whole process work or to clean or to dry. So that's one of the things that we think about from a design perspective.
But again, this sump is generally filtered to remove insoluble particulate. We'll have a circulation pump that turbulates this sump. It suspends particles in the flow of fluid which is drawn into the pump suction, and those entrained particulate may be captured on the filter element and therefore removed from the process. Again, ultrasonics can be used to enhance cleaning in this regard for parts that would benefit from this type of process.
Additional Components of Vapor Degreasers
Patrick Oliver:
Here are some additional components of the system. We talked about the, for example as I mentioned, thermal capacity, there is a feature of these systems, a technical specification if you will, that we assign to the systems. They're all rated to process a given number of pounds per hour. In terms of placing baskets into and out of the degreaser, we want to control that motion. In other words, if you put a basket in too fast, or pull it out too fast, you could create a draft that could potentially displace vapor.
When the basket is in the neighborhood of 50% of the vapor volume, volumetrically speaking, dimensionally speaking, that speed must not exceed 11 feet per minute. There are some cases where we've got systems that the basket may almost approach that at of tank opening in terms of its horizontal area. In those cases, we actually slow it down to three feet a minute. So 11 feet a minute sounds like it's a lot, but it's not, it's fairly slow, but we regulate all of our programmable material handling systems to operate at less than 11 feet per minute.
To the next point, the freeboard area, and this is again the uppermost set of coils. Beyond providing additional efficiency in confining the vapor in the degreaser, that super cold air there can be beneficial in recovering minute amounts of solvent or solvent vapor that may be localized around part surfaces like in the internal diameter of a tube, porosity, blind holes, things like that. So oftentimes it's not unusual after the part has allowed to, or been allowed to dwell in the vapor until drying occurs and condensation is observed to stop, the part may be paused in the freeboard for final recovery of minute amounts of solvent that might otherwise escape the process. So again, another efficiency feature of the freeboard.
The cover is used to prevent vapor loss. When you are not processing you close the cover. And oftentimes, when we have a programmable material handling system, the cover will open to allow the hoist to approach, run the process, when the hoist extracts the cover closes. We've even got some designs where the cover can close around the mast of the hoist. Sometimes we have enclosures around machines, sometimes we have vacuum degreasers. So there's different ways to isolate the solvent from the environment, but even the most basic degreasers all have a sliding cover.
And then of course, the water separator. How does water get into the process? Well, the fact that you've got two sets of coils running well below the dew point in the room, you're going to draw some moisture in the air. The uppermost set of coils running at minus 20 Fahrenheit naturally they're going to frost, so we defrost. And that moisture which does not evaporate in the defrost cycle accumulates in the degreaser and it's removed by the water separator. If you don't remove water from the process, you can create an aerosol of water in the vapor, and the vapor will look hazy, foggy, white, like a pond in the morning. It looks like fog mist.
And what can happen is you can remove parts from this hazy, misty, watery vapor, and the water spots can remain on the part surfaces after the solvent evaporates. It can cause water spotting, streaking, staining visual defects. Not to mention there's a significant difference in the specific heat of the water versus the solvent, so the water is going to compete thermally for the available energy in those regions. But for the sake of efficiency, consistent processing, you definitely want to get water out of the process.
It's very easy to do. The water separator, you open a valve. And even on the largest of degreasers, you're collecting a relatively small amount of water. This degreaser here that's depicted in this cutaway, the overall footprint of this thing is about the size of your desk, and you might get an ounce of water out of there a day. If you're operating in an air-conditioned environment, very little water. In the summer where dew points are higher, you may get a little bit more. But generally, that amount of water that comes out is minimal. The water must be disposed of in accordance with local regulations. It's essentially pure water, but there could potentially be a few part per million of solvent in it. But in terms of managing water effluent from a vapor degreaser as compared with an aqueous cleaning process, it's negligible. I mean, you're talking a few drops of water, honestly.
Are Foggy Vapors an Indication of Excessive Water in the Vapor Degreaser System?
Kevin Pawlowski:
Hey, Patrick, you brought up an interesting point which it was new to me. So it's great, I'm learning things as well. Is if you get a hazy appearance in the vapor zone, is that symptomatic, is that a general symptom of too much water in the system?
Patrick Oliver:
Absolutely. And it can happen when the water separator is not empty. In other words, what can happen... And it's counterintuitive, because again, the difference in density between water and the solvent. These solvents are probably 10 and a half pounds per gallon, maybe for some of the fluorinated stuff. Okay, whereas water 8.375. The water is going to float on top. But if you allow enough water to accumulate, and we've seen this, a cap of water can form in the separator and eventually force the solvent level down and the water can flow into the degreaser. It'll get into the immersion sump, it'll get into the boiling sump, and then you kind of get this aerosol of water going. It's the entering the vapor to some extent, I guess by partial pressure, but it's also that rolling boiling action in the boiling sump creates an aerosol and the water droplets get suspended in the vapor. And when you look at it looks just like fog.
And some of the legacy solvents, trichloroethylene, perchloroethylene, especially n-propyl bromide had the potential to form their corresponding acids: hydrochloric, hydrobromic acid, if the water causes a hydrolysis reaction. And this can be devastating. You can have acidity corroding the degreaser. You can have corrosion on parts. With the modern fluorinated solvents it's really not an issue, but again, it can be a problem having water in your vapor. As I said, this can cause streaking, staining, spotting, and it also reduces the overall efficiency of the degreaser in respect to vapor generation, distillation rate. But if you can devote literally 10 to 30 seconds a day, you will not have water in your vapor degreaser. It's just that easy to drain it off.
Auxiliary Cleaning Options of a Vapor Degreaser
Patrick Oliver:
Okay, so we get into auxiliary cleaning options. As I mentioned ultrasonics, which I think a lot of people are familiar with, does provide sort of a scrubbing action that enhances the cleaning process. Ultrasonics are available in a variety of frequencies with the lowest frequencies being the most powerful, the highest frequencies being most gentle, if you will. Normally we are sizing to a watt density of 40 watts per gallon. So the ultrasonic energy, we try to hit that target of 40 watts per gallon in the immersion. And our standard frequency is 40 kilohertz, that's normally what we use. Although for heavier metal cleaning, more difficult cleaning, we may go down to 25 kilohertz, and then higher frequencies can get up to 132 kilohertz is pretty much where it goes. But we've also got some selectable frequency where you could have like 40-80, 40-80-120, in case you have the need to try different frequencies to see what works the best.
The offset boiling sump. You can actually have a degreaser with more than two sumps. Sometimes the boiling sump is offset. In other words, if you look down into the tank, you don't see the boiling sump, it's offset and the vapor enters through a slot in the wall. There's a couple reasons you may want to do this. In other words, you may want a precision process where you eliminate the ability for the operator to place baskets in dirty solvent. The offset boiling sump accomplishes that objective, or you could create a degreaser with multiple immersion sumps. Each successive immersion sump is cleaner than the sump proceeding it because it's got more distillate, more pure solvent in it, lower contamination. So more sumps can equal more cleaning, but again, this is certainly the most common configuration. One boil sump, one immersion sump.
Manual spray wand as we discussed, we offer that on a lot of degreasers except the very small units. And as the other attendee asked, yes, it can increase emissions so you have to kind of be wise how you use it. Sometimes people rely very heavily on the manual spray want, and for this reason, we provide increased capacity distillate receivers. This is the tank that holds the fresh clean solvent that the spray pump is supplying to the wand. And your spray time is a function of the flow rate of the pump, the distillation rate of the degreaser, So sometimes you need more spray.
Heated condensate sump, sometimes we do heat the clean sump in an effort to reduce the cycle time, warm the part closer to vapor temperature to minimize the amount of time it must dwell for drying purposes. And sometimes we even cool the sumps. Sometimes we'll have submerged evaporator coils that can chill solvent in downtime modes.
Will Over Reliance on Spray Wand Add Variability to a Cleaning Process?
Kevin Pawlowski:
You had mentioned those that rely on the spray, the manual spray, which is interesting to me because if as part of the process that they're relying on it over and over and over again, you're removing something that's a very reliable, very low variability process and adding variability to it. So it would behoove you if you do rely on this to reach out to Oliver, to Pierce on the solvent side, to an expert to really take a look at your process, take a look at your solvent, see if there is a better way to do it, just to strip out that variability in your process.
Operational Enhancements of Vapor Degreasers
Patrick Oliver:
And then we go to operational enhancements. A desiccant is typically a molecular sieve used to chemically absorb water. Sometimes there's cases where degreasers are operate in very humid environments, it's difficult to control the humidity, or certain formulations of solvent may contain an alcohol. For example, in the defluxing of circuit boards or printed circuit boards, electronic assemblies, oftentimes alcohol is added to help dissolve some inorganic components of those soils. And the alcohol will actually dissolve in the water which accumulates in the water separator. So when you drain the water, the alcohol comes out too. The desiccant can slow this process down and prevent the extraction of alcohol.
But desiccant only provides benefit to that extent which it is maintained. In other words, most people have their desiccant split into two parts. One half is baking constantly in an oven to dry, the other's in the degreaser, then you switch. But it takes a lot of desiccant to adsorb a very small amount of water, so it's very commonly used again in electronics type applications.
Filtration, especially of the immersion sump, again, to remove insoluble particulate. And we've done filtration that absolutely runs the gamut. Even absolute filtration where you get into like 0.22 micron or less for medical or other half precision type applications. The secondary subzero freeboard coils, definitely used in larger degreasers or for enhancement of efficiency, etc. An important feature there.
Programmable material handling. There's a lot of ways to automate this process. Some are very simple, some can be quite sophisticated interfacing with accumulation conveyors. We've got rotational processing, monorail, vibratory, I mean, you name it, there's a lot of material handling options, which increase efficiency and consistency and redirect labor. So just about any degreaser, even our small bench top degreaser, we offer a material handling system which is programmable.
Airless Degreasers
Patrick Oliver:
The vacuum degreaser essentially performs the same basic process functions as the open top, the main difference is that the entire process occurs in a vacuum chamber. In other words in an open top degreaser, you're basically bringing the parts to the solvent, whereas in a vacuum degreaser we bring the solvent to the parts. The solvent is stored in sealed vessels and the product is placed into a chamber, think of like an autoclave or you may be familiar with vacuum oven, vacuum braising oven.
Essentially, we put the parts in a vessel and close the door, we pull a vacuum and we pull it down to nearly one tor. And essentially, when the parts are under vacuum there's no air in between the solvent and the substrate. To a certain extent, air can be a barrier that may present or prevent the solvent from coming in contact with the part surface. Imagine if you had a blind hole, in other words, if you can't displace the air from the interior of that hole solvent can come in contact with it. But in an airless degreaser, we pump all the air out. So when we introduce the solvent, either as liquid or vapor, it rushes in to fill all of those spaces. It can get into porosity, blind holes, capillary, the internal diameters of very long, very narrow tubes, honeycomb that's used in aircraft structures, it comes in intimate contact with every space on the part. The surface underneath a BGA on a circuit board, it gets right in there.
And then in this type of process, you can expose the parts to vapor spray, immersion, ultrasonics, and rotation, rocking, tilting, whatever you need to do. At the end of the process, we drain out the liquid and then we pull another vacuum. So under vacuum, there is some residual solvent in the chamber on the part, but at the vacuum that we're operating, the boiling point of the solvent is dramatically decreased, the vapor pressure is dramatically increased. And essentially, the latent heat of the part flashes off the solvent and it dries. So you're getting nearly complete total recovery of the solvent.
These systems also provide continuous distillation. And they have a means where you can boil the solvent down and recover just about 100% of the oil and grease with virtually no solvent in the waste stream. In fact, these systems are so efficient, they're generally acceptable for use anywhere in the world, regardless of whatever regulations may be in place locally, certainly federally. And these systems can be used with a variety of different types of solvents, halogenated, hydrocarbon vapor degreasing solvents, as well as aliphatic hydrocarbons, petroleum distillate type materials, alcohol like isopropyl alcohol, acetone, as well as some of the modified alcohol chemistry.
So chamber size on these can be very small up to extremely large. Some of the largest vapor degreasing equipment in the world is vacuum. So there's a lot of options here. And this has by far and away the most minimal impact to operators, to environment. And in many cases emission's so low they're virtually immeasurable. So solvent savings running something like this is dramatic. These systems are very, very efficient.
Vapor Degreaser Unit Location
Patrick Oliver:
Obviously, we normally like to have like a 24-inch envelope around the system for ventilation, maintenance access, operator access. We want the system to be level because we're relying in many cases on gravity, certainly for the open top degreasers, so we want everything to be predictable in terms of the level.
The final point is probably the most important. We don't like drafts with open top degreasers. You would not want to install these near open bay doors, fans, HVAC ducts, areas where there's lots of traffic, forklift or whatever passing. Again, if you create a draft over the top of an open top degreaser you could potentially displace the cold air blanket, and maybe displace vapor. So we like to minimize drafts.
Vapor Degreaser Startup & Initial Charge
Patrick Oliver:
This basically describes how you power up a degreaser from start. Obviously, you're making sure all the valves are closed. We turn on the main power, the refrigeration system comes on. And then essentially what's happening is you're cooling the air above the area where the solvent will be placed to minimize evaporation when you're transferring the solvent initially to fill the degreaser.
Grounding connections: the system is grounded through its electrical enclosure. And then we're adding solvent to the recovery sump, it overflows to the boiling sump. And then once you turn the heaters on and the process begins, the interstitial volumes of piping will fill, the distillate receiver, the water separator. And the level in the boil sump may drop a little bit, and then so you add a little bit more back to the boil sump to bring it up to operating level, which is normally maybe two inches below the weir separating the boil and immersion sumps. Close the cover. Let the system come up to temperature. We have a control that we call vapor up, which indicates ready to process. When the vapor up condition is met, you can start cleaning parts.
Starting Vapor Degreaser Heat Unit
Patrick Oliver:
And essentially, this is basically how you begin heating the degreaser initially to start up the process. We've got temperature, we're measuring temperatures all over the machine. We're comparing those to set points to make sure all proper conditions are met. And the degreaser will create an alarm condition if there's any problem with that, just to make sure that everything's operating safely and predictably. And again, we set temperatures based on the boiling point of the solvent. So that's how we establish control.
Vapor Degreaser Boil Sump Maintenance
Patrick Oliver:
The boil sump maintenance. Okay, eventually, you're going to saturate the solvent in the boiling sump with soluble contaminants. Normally, when the temperature in the boiling sump elevates approximately five degrees Fahrenheit above the boiling point of the pure solvent, it's normally considered to be saturated to about 30% by weight with soluble oils. That's a lot. Now, when you add soluble contamination to any solvent, the boiling temperature elevates, this is how we know that the soil or that the solvent in the boiling sump is saturated with soils.
So in this regard if you create a too high of a ratio of soil to solvent the boiling temperature elevates, it takes more energy input to generate vapor. The efficiency goes down. Not to mention there are some soils, oils and greases that could become combustible. So we don't want to create a situation like that where you could have an issue where the soil could catch on fire. So we've got all these safety controls in place to prevent that.
So when you get to the saturation temperature, you turn off the heater. You can let the sump cool down, and that's when you can drain out the soluble soil residues that are entrained in the solvent and the boiling sump. Sometimes we enhance this process with a secondary distillation system where you're removing all this contaminant continuously online and maximizing production. So we have ways to keep the system running and run production simultaneously. But my point here, compared to other types of cleaning processes where it may be difficult to determine the viability of the chemistry, in vapor degreasing it's very easy to monitor that simply based on temperature. And the system does it basically automatically, it will alarm you, alert you when you got to pay attention to it.
Vapor Degreaser General Maintenance
Patrick Oliver:
And this pretty much right here, what you're looking at right here is basically the sum total of maintenance requirements on a vapor degreaser that you do most frequently. Again, draining the water separator takes literally seconds, you do it at the end of every day or every shift. That way the system has been running, distilling, accumulating all the water in the separator so you can drain it off. And it keeps the system healthy and happy, basically.
Some solvents require an acid acceptance test. Generally these are legacy solvents like trichloroethylene, perchloroethylene, n-propyl bromide also know as nPB. Essentially, you're monitoring the stabilizer content that prevents the solvent from becoming acidic. That's a simple titration and it takes about 10 minutes, but you got to do it. Normally, we say at least every two weeks, but basically as recommended by the solvent manufacturer. And the solvent manufacturer can provide the acid acceptance test kit and stabilizer to dose the solvent to return it to control if necessary.
Filters. Again, we're removing particulate and when the filter element becomes loaded it must be changed. Often this is done on a preventive maintenance schedule. Some equipment is equipped with differential pressure transducers or gauges that can let you know if the filter element's getting clogged. Very simple to maintain. And-
Vapor Degreasing Solvent Loading
Pierce Pillon:
When your solvent is loaded, essentially what's happening is your solvent is reused over and over, and you're adding makeup as you go along and so it's topping off from your operators just due to normal solvent loss. And when it becomes loaded, that just means that all the contaminants are increasing your heat load, it's not cleaning like it should. There may be other aerosolized-type contaminants such as water like Patrick alluded to earlier. And at some point, it's not going to meet your cleaning requirements. It's just essentially going to quit cleaning like you want it to.
So this needs to be trended based on your chemistry, your equipment manufacturer recommendations. Patrick mentioned five degrees Fahrenheit. That varies with the temperatures. Some go to five degrees C over the normal boiling temperature. It depends on the chemistry, depends on where the set points are for the high alarms, things of that nature, also the soils that you're dealing with. Your normal boil down or change-out schedule, that's just recommended so you stay on top of it, and you don't see a noticeable loss in your cleanliness inspections at the end of the operation.
So basically, to boil it down, you just run the vapor degreaser, and you capture the clean solvent. You just run all of your boil sump essentially dry or almost dry. And that's how you do it. So, the material can either be reclaimed through distillation or sending it off site to be reclaimed. Or it can be replaced with fresh material right off the bat. And then the dirty material just disposed of according to the local regulations and the permits that you operate under.
When Do I Have To Replace the Vapor Degreasing Solvent?
Kevin Pawlowski:
Pierce, I have a question, but I'm going to answer the question. So just because it's pretty straightforward. So, "Generally, how many cycles my solvent will saturate before I have to replace it?" It's going to be so dependent on so many variables, on how many cycles you can go through before you have to replace your solvent. So if there's any question, definitely feel free to reach out to Pierce, and he can kind of talk you through the process on how to build that up as a standard operating procedure to keep an eye on that. Does that sound like a fair answer, Pierce?
Pierce Pillon:
Yeah, it basically comes down to your throughput. And if your throughput is consistent, you can trend that. If it's very inconsistent, then it's going to be difficult to get any historical data to trend. So, but we can... They're more than welcome to contact me. Patrick is a great source also. So either one of us can hopefully, at least get you in the ballpark.
Selecting a Vapor Degreasing Solvent
Pierce Pillon:
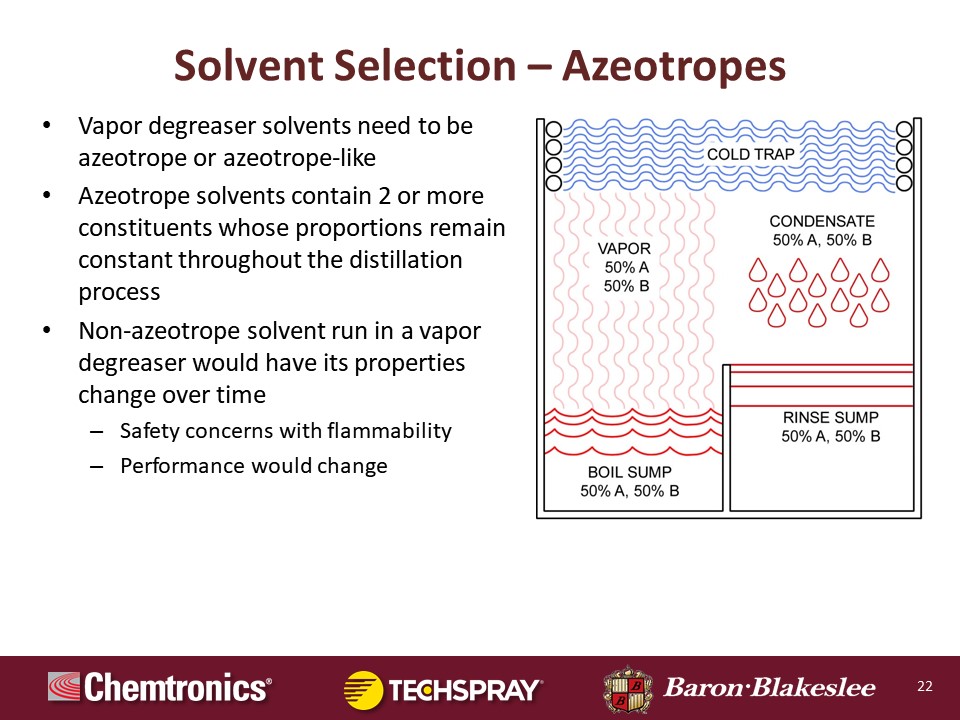
As far as solvent selections, essentially, you have two types of chemistries azeotropes and nonazeotropes. So it's always recommended to either have a true azeotrope or what has come to be known as a near azeotrope. And what an azeotrope is, it's a formulation of two or more components, and they do not fractionate, okay? They will start off with different boiling points, but when they're mixed into proper proportions they actually have one boiling point and it does not fractionate. And as you can see what an azeotrope does, if you have 50% of A and 50% B, you're going to maintain that same composition from the boil sump to the vapor phase to the condensate phase, from the rinse sump, and then from the rinse replenishing over into the boil sump. It does not change, okay? That is a true azeotrope.
Now, near azeotropes generally can fractionate a little bit and they will trend, they'll skew a little bit from one end to the other on the compositional mix, but they remain very, very stable once they do that. Now a totally non-azeotrope is going to fractionate, and so your lowest boiling material that's what's coming off first. And depending on what the percentage is in the formula with the rest of them, you may end up with some flammable material in your system, whether it's in the vapor phase, whether it's in the liquid phase. So it's very important for safety that you know what you're dealing with and make sure that you quiz your chemistry vendor very, very thoroughly because you want to make sure that you're getting the right material and the right equipment to do the cleaning that you need to do.
Solvent Chemistry Challenges
Pierce Pillon:
Okay, the challenges to chemistry. Well, there's all sorts of agencies that have their fingers in the pie. You've got federal agencies, you've got state agencies, you have local municipal agencies. And as long as a lower category of agency... How do I want to put this? For a state, a state cannot get any less restrictive than the feds. They can be more restrictive, but they cannot be less restrictive. And so that's why you see some of the air quality districts are CARB, some of the agencies in California, for example, they're very, very restrictive compared to the federal regulations. And we have to meet all of those depending on whether or not we want to sell them in that area.
And so over time, the chemistries have changed that we can offer to the customer. The ozone depleters were pretty much completely phased out at the end of 2014. The CFCs were gone years and years ago. The HCFCs like 141b, AK-225, those are gone. The HFC, the hydrofluorocarbons are now coming under pressure due to the global warming potential. Most people think of HFCs as the propellants in an aerosol. Well, that's true. But there are a lot of HFCs in the formulations in vapor degreasing products. Just two of them off the top of my head is HFC-4310, which is commonly called Vertrel XF, that's under pressure. HFC-365mee, commonly called SOLKANE, which is a Solvay product, that's under pressure. They are both hydrofluorocarbons. So we'll see how that's going to be restricted.
The chlorinated solvents: TCE, perc, they have been over many years tighter and tighter regulated under various use conditions. And that's due to an environmental impact from disposal to spills to whatever. Also the toxicity in the immediate area for the operators and the bystanders. Brominated solvents like nPB. They've been under pressure for a long time due to toxicity and now the government is finally looking like it's going to act. And I'll get into that in the next slide.
Toxicity Concerns of Vapor Degreasing Solvents
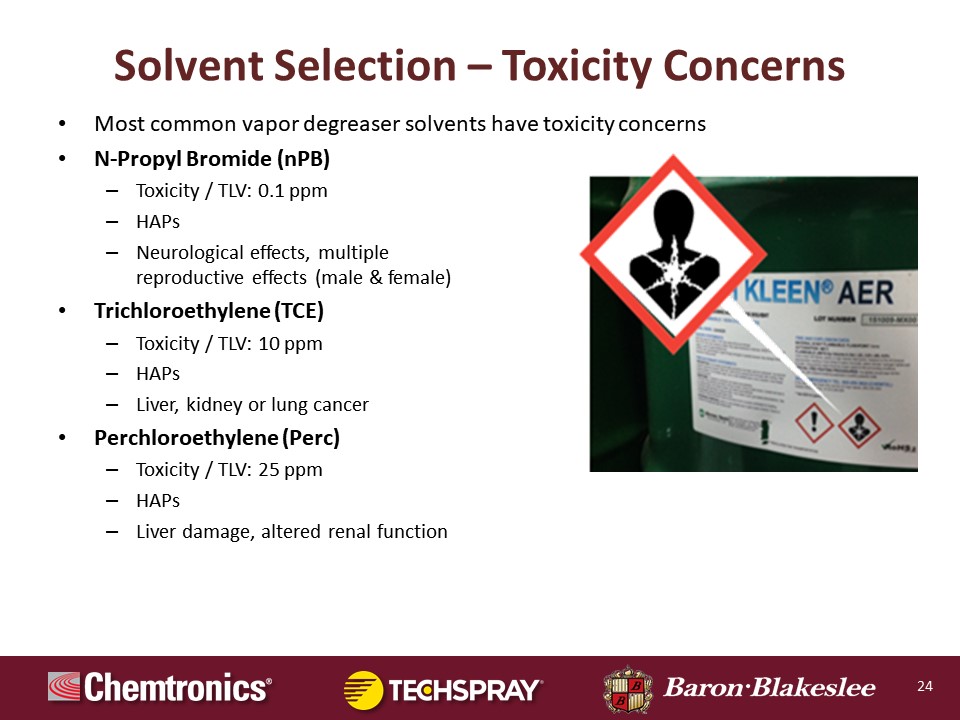
Pierce Pillon:
Okay, here we go. Speaking of TCE, perc and nPB. These are very common vapor degreasers. They're inexpensive and they work. That's the bottom line for a lot of operations. Just hitting on TCE and perc, you can see that the TLV is 10 ppm, 10 parts per million for TCE. Perc is 25 ppm. They are both considered by the government as toxic or what they call air toxics, which means that they are actually listed on the Hazardous Air Pollutant List.
Now I would like to spend the remainder of the time here on nPB. It's been around for many, many years. In 2014, the ACGIH, which is the American Congress of Governmental Industrial Hygienists finally lowered their TLV value from 10 ppm to 0.1 ppm. That's extremely low. Now, there's a variety of reasons for this. There's various neurological effects. There's male and female reproductive effects. Most of these are chronic. The neurological effects can be acute. When you see if you see over in that diagram to the right, this is one of the new GHS pictograms, that's what's commonly called the exploding chest. That can be anything from cancer to other various neurological effects. nPB has that pictogram.
Vapor Degreasing Personal Protection Equipment (PPE)
Pierce Pillon:
Okay, personal protective equipment. If you can smell it, it's escaping from your unit. That's essentially why you need to protect yourself or your workers. As Patrick said, if you have a well-maintained machine, it's going to be extremely minimal. However, most of them are not, or many of them are not. Their preventive maintenance is not performed. There's just a lot of reasons. The gloves, the glasses, the face shield, the respirators if necessary like the half face masks, sometimes those are necessary. The biggest deal that you can do for your employees is to make sure that you keep your EHS team or your safety team in the loop with your vapor degreasing operations, and any complaints that your operators or bystanders may have.
Chemtronics Vapor Degreaser Solvents
Pierce Pillon:
Everything in the formulations on solvents, on the vapor degreasing solvents outside of performance is driven by the cleaning requirements of the customer and essentially by regulations. The wish list includes high performance, low VOC (the volatile organic carbon content), and the low global warming potential. Those options are out there. Most of the materials that are on the market today have a good record of this. Chemtronics has the Tri-V line, that is the Electro-Wash Tri-V, the Flux-Off Tri-V. Those are designed as nPB replacements. They are very high performance. The Max-Kleen Tri-V is a near azeotrope. It's highly stable. It's actually recommended for if you're going to have a side distillation system to use that.
Techspray has the PWR-4 line which are similar to the Tri-V line. They were also designed as nPB replacements. Techspray also has the Precision-V line, and they are very well used for sensitive materials and things of that nature, regulatory compliance, and they are also high performance materials. And to help you decide where you need to do that to Techspray and Chemtronics has an in-lab qualification team. We can run your materials, we can simulate your materials with the same soils, and do cleaning trials for you. We'll help you in any way necessary.
Some final words. Relationships matter, they absolutely do. And the best relationship that you can have is between your operations people and the equipment manufacturer and your chemistry supplier, both for training and maintenance of the equipment and any challenges any changes in your operations that may need changes in the parameters of the process operation, and any new solvent the qualifications that you may need to go on as your needs change.
How Can You Avoid Material Compatibility Issues with Vapor Degreasing Solvents?
Kevin Pawlowski:
Okay, so starting at the top, we do have a question that's very specific to materials compatibility. "Are there circumstances where it can react to glue adhesives? Is that an issue?"
Patrick Oliver:
Depends on the glue. I mean, we've got some cases where people are using the vapor degreasing process to strip off glue. Sometimes glue is removed unintentionally, because it's incompatible with the solvent. And there must be a million glue formulations out there. So I think it needs to be looked at from a case by case perspective, but there's lots of materials we're cleaning that are laminates or assemblies or electronic in nature that got glue. And the right solvent, right glue combination, it's not a problem. If there's a chemical incompatibility, it can damage or remove the glue.
Kevin Pawlowski:
And so yeah, like Pierce, for example, the solvent supplier contact would be a good contact for that. If you think about it, the solvent doesn't necessarily know the difference between the soil and the substrate. And so you've got to dial that solvent in to where it is a good match for your substrate to where it attacks the soil not the substrate. So your glue is that you want to stay put.
Should I Be Concerned About Vapors Escaping the Vapor Degreaser Unit?
Kevin Pawlowski:
"So what do you suggest if the freeboard coil does not have a negative 20F, and perhaps has a 70F? Should we stop using equipment right away?" So apparently, there's the older equipment that is not keeping the solvent in place. So there's actually coworkers that are noticing the odor on the floor. So, Patrick, what would be your recommendation for a situation like that?
Patrick Oliver:
That could be a real issue, okay? It depends on what solvent you're using. I mean, you may be exposing your operators to a level beyond that which is permissible or recommended. Obviously, you may have an air permit that's impacting, and then you're going to be replacing the solvent which is evaporating, which is going to cost you money.
Typically, a local certified licensed refrigeration technician can repair that freeboard refrigeration system for you. It may simply be an issue with the refrigeration system, it could be a control issue. Now this is not a guy that works on air conditioners, or someone who works on air conditioners. More like an industrial process refrigeration technician, typically somebody with low temperature experience can fix that. But I don't know where you are, but if there's somebody in your town that can fix it. Or if it's a Baron Blakeslee call us and we'll do what we can to guide you through it and help you any way we can. But that is a problem that needs to be addressed immediately. Yes.
Can Vapor Degreasing Solvents Become Oxidized?
Kevin Pawlowski:
For azeotropes, have you experienced situations where a component of the azeotrope becomes oxidized? If so, what's the common causes for this?" And I'm not familiar with that. Pierce, you have anything?
Pierce Pillon:
I would tend to say it's because you're getting air entrained in your system. I don't know what could be oxidized just off the top of my head, knowing some of the degreaser formulations that are out there.
Patrick Oliver:
Is that used with alcohol? I mean, I know there's some processes out there where they have a cyclohexane isopropyl alcohol azeotrope. Maybe the IPA is coming out in the water separator, and that's not an azeotrope anymore.
Pierce Pillon:
Right, it's not balanced anymore. I mean, without knowing a little bit more about it, it's just, it's too difficult to tell them, and there's a lot of things that could go wrong.
Patrick Oliver:
This is probably where a desiccant could be beneficial, or maybe maintaining your desiccant would help. Again, we all need more information.
Kevin Pawlowski:
So that's all the questions we had. Great job for on our speakers: Pierce and Patrick. Thank you very much. If you have any more questions, please reach out to any one of us, and we're going to be happy to get into deeper detail. All right. Thanks, everybody.